Hydrocarbon Solvents
Sunday, March 13, 2011
When I was a project/chemical engineer working in the oil and gas industry 6 years ago, one of my critical tasks is to manage the specialty chemical matters. This is an area which requires a person with chemistry or chemical engineering background. I was fortunate because the company I worked with is attached with a well known and established international specialty chemical manufacturer. Hence, I learned a lot of chemicals and its formulation from the principal company. There are various specialty chemicals available for numerous industrial applications such as corrosion inhibitors, degreaser, scale inhibitors, descaler, deoiler and others. Among the chemicals that I frequently handled is the corrosion inhibitor. The corrosion inhibitor is used in the internal pipeline chemical cleaning project awarded to us to lengthen the lifespan of the gas  and condensate pipelines from corrosion threat. The corrosion inhibitor concentrate, which comes in drums of 200 liters will be then blended with either water or hydrocarbon solvents to the desired concentration. The chemical blending was made on-site by the staffs. After the blending is completed, I will check the final corrosion inhibitor blending via bottle and emulsion test. Blending the chemical using water is not as complicated as using hydrocarbon solvents.
and condensate pipelines from corrosion threat. The corrosion inhibitor concentrate, which comes in drums of 200 liters will be then blended with either water or hydrocarbon solvents to the desired concentration. The chemical blending was made on-site by the staffs. After the blending is completed, I will check the final corrosion inhibitor blending via bottle and emulsion test. Blending the chemical using water is not as complicated as using hydrocarbon solvents.
When blending using hydrocarbon solvent, I need to identify which solvent best suits the chemical. I also need to consider the economic as well as the safety aspect. Hence, for me, there is an importance to know about hydrocarbon solvents. One way to learn about it is from the material safety data sheet (MSDS). Besides that, I also always refer to the notes and my principal for further explanation or analysis.
Basically, there is a wide range of hydrocarbon solvents which include aliphatics, paraffins, aromatics, blends, mineral spirits and others. There are also daily used items that relies on hydrocarbon solvents such as adhesives & sealants, paints & coatings, dry cleaning fluids, insecticides, edible oils and tyres. A simple and common example is when we mix kerosene into the paint concentrate when we paint the walls of our house. Kerosene is one type of popular hydrocarbons, which is also the fuel for aeroplanes.
On top of that, other application of hydrocarbon solvent includes cleaning or dissolving water-insoluble substances such as greases and oils. Due to their high toxicity and persistence in nature, their use in industrial processes is restricted. The solvent must also be stored safely in a dry cool shady place and well ventilated area, far from heat and sunlight. This is to avoid them reaching their flash points which may lead to possible explosion.
It was really nice experience learning and being exposed to the specialty chemicals and various hydrocarbon solvents. It is indeed a huge market and a profitable area to be in. Don't you think so?
Labels: Chemical, Chemistry, Environmental, hydrocarbon solvents, Learning Curve, Oil and Gas
posted by Kipas Repair JB @ 12:43 AM,
,
![]()
Chemicals information at ThomasNet
Monday, January 31, 2011
Thomasnet is an industrial search engine that provides one source for finding the exact product, service, or supplier –quickly and efficiently. ThomasNet provides direct access to the detailed information needed to make a purchasing or specifying decision, including line-item product details, CAD drawings, and more.
 Chemicals are produced through a combination of reactive elements, and can be found in solid, liquid, or gaseous states. The term “chemical” refers to a vast number of substances and processes that cannot be fully addressed in a single comprehensive manner, but by focusing on those in the pharmaceutical and industrial fields it is possible to derive some general information. In natural or artificial forms, these specific chemicals play a crucial role in the production of industrial components and medicines. Although chemicals are common in day-to-day life, proper care must also be taken when handling them, particularly those with the potential for harmful or volatile reactions.
Chemicals are produced through a combination of reactive elements, and can be found in solid, liquid, or gaseous states. The term “chemical” refers to a vast number of substances and processes that cannot be fully addressed in a single comprehensive manner, but by focusing on those in the pharmaceutical and industrial fields it is possible to derive some general information. In natural or artificial forms, these specific chemicals play a crucial role in the production of industrial components and medicines. Although chemicals are common in day-to-day life, proper care must also be taken when handling them, particularly those with the potential for harmful or volatile reactions.Chemicals are integral to modern society, whether in medicine, industrial manufacturing, or the production of foods, glues, and plastic. Chemical substances can be broadly categorized as metal, non-metal, compound, element, and covalent types. Manufacturers have used the chemicals in these categories to not only create products, but also a wide range of specialized professions, such as engineers, scientist, and chemists.
Thomasnet.com one place to find out about everything related to chemicals and chemical related information. At thomasnet one can find for Chemical related articles, Chemicals suppliers, chemicals specifications and chemical product catalogs for any kind of Chemicals at Standard and Custom blended Chemicals. Thomasnet has recently posted new Need-to-Know guides for Chemicals related topics at http://www.thomasnet.com/
Photo credited to: http://www.greenplanet.com/we-are-what-we-eat-and-processed-food/




Have you downloaded my free "Choosing Alternative Fuel" Ebook? If not, then please download it here. It's Free and on top of getting the free ebook, you'll get eCourse on Alternative Fuel. It's a good and easy way to add more valuable information to yourself.
Labels: Chemical, Chemical Engineer, Chemical Engineering, Technology
posted by Kipas Repair JB @ 9:59 AM,
,
![]()
Four Basic Engineering Related Trainings in October
Saturday, October 30, 2010
Followings are the trainings that I have recently attended in the month of October.
1. Engineering Code of Ethics (12 CPD hours) 4 & 5 October, IEM Building, PJ.
2. Engineering Management Practice (12 CPD hours) 19 & 20 October, , IEM Building, PJ.
3. Safety and Health at Work (12 CPD hours) 25 & 26 October, IEM Building, PJ.
4. Engineering Failure Analysis (7 CPD hours) 23 October, Faculty of Mechanical, UTM.
All of the trainings mentioned above have certain fees to be paid and that is part of the commitment to be and maintain ourselves as a professional engineer.
During the Engineering Failure Analysis training, on the tea break, I have a chat with Assoc. Prof. Dr. Ir. Shuhaimi (who also is a committee member for the IEM Southern Branch) from Mechanical Engineering Faculty, UTM. He told me that an engineer chatted with him about him spending more than RM4000 already that year just to attend trainings to maintain his CPD hours and maintain his title as a professional engineer. The cost includes training fees, fees, food, accommodation etc.
Basically, this message shows that to maintain professional engineer status it requires certain amount of cash. It is very good that engineers attend training to strengthen and enhance their knowledge. It is also good that as engineers, we can learn other disciplines, information, field of engineering. On top of that, we can mingle around and do networking with other engineers.
I personally agree with the fact that all professional engineers need to attend trainings and courses, technical visits and tours. I can say this because I have personally experience it and felt the benefit. I am exposed to more engineering and industrial information besides learning some of it from Discovery channel!!!
I also managed to be friends with several dozens of engineers from various disciplines, background and industries when I attended those trainings. I have more contacts and I strongly believe that is beneficial for me. It is good for me to have new civil, electrical, mechanical and chemical engineer friends. Off course, we exchanged our name cards and other contact details.
Interestingly, with reference to the mandatory courses that I attended, I discovered that there were those of the age of 36 to 48 attending the course. These people, despite of their age, still have the desire to get an engineering degree (which obviously they earned it) and pursue their own quest to be professional engineer. I have full respect of them... These people still have the will power and desire to get themselves chartered and recognizable.
So, regardless of your age, get yourself chartered or have the professional engineer status if you still don't have it. If you are still young (just recently graduated), go for it. Get the right information, get yourself registered and follow all the necessary training program. Don't delay and hesitate. Time will not wait.
If you want to learn more about IEM, click here.
If you want to learn more on the trainings and courses organized by IEM, click here.
If you want to learn more about IEM Southern Branch, click here.
If you want to learn more about BEM, click here.
-------------------------------------------------------------------



 Have you downloaded my free "Choosing Alternative Fuel" Ebook? If not, then please download it here. It's Free and on top of getting the free ebook, you'll get eCourse on Alternative Fuel. It's a good and easy way to add more valuable information to yourself.
Have you downloaded my free "Choosing Alternative Fuel" Ebook? If not, then please download it here. It's Free and on top of getting the free ebook, you'll get eCourse on Alternative Fuel. It's a good and easy way to add more valuable information to yourself.Labels: Chemical, Chemical Engineer, Chemical Engineering, Experience, Health, pH.D, Seminar, TQM, Training
posted by Kipas Repair JB @ 9:03 AM,
,
![]()
Outsourcing Chemicals for your work
Wednesday, May 19, 2010
 I can still recall when I prepared the technical tender documents and performed bottle test for several chemicals/resins in few South China Sea Petronas offshore platforms, I was exposed to new chemicals. A lot of them were very complicated and difficult to pronounce or to write. Not just that, for other project, I have to formulate our own chemicals and blend them to be a good soluble solution which can dissolve in petroleum condensate in order to clean up the stubborn internal petroleum and gas pipeline scale and fouling. Well, that was for a downstream project which I lead few years ago. It was really interesting and challenging experiences. As I was in charge mainly on chemical department, in order to do that, I needed to prepare few chemicals and constantly consult with our international partner. Our principal provided superb advice and support. However, there were times where they asked us to outsource for chemicals locally as they were cheaper and immediately available compared to importing them from overseas and messing up with the tedious lengthy custom process.
I can still recall when I prepared the technical tender documents and performed bottle test for several chemicals/resins in few South China Sea Petronas offshore platforms, I was exposed to new chemicals. A lot of them were very complicated and difficult to pronounce or to write. Not just that, for other project, I have to formulate our own chemicals and blend them to be a good soluble solution which can dissolve in petroleum condensate in order to clean up the stubborn internal petroleum and gas pipeline scale and fouling. Well, that was for a downstream project which I lead few years ago. It was really interesting and challenging experiences. As I was in charge mainly on chemical department, in order to do that, I needed to prepare few chemicals and constantly consult with our international partner. Our principal provided superb advice and support. However, there were times where they asked us to outsource for chemicals locally as they were cheaper and immediately available compared to importing them from overseas and messing up with the tedious lengthy custom process.This was when I normally look out for local chemical manufactures and get the best deal from them. Miraculously, they have in their stock weird and complex chemicals that we need. Hence, it is good to work with reliable and resourceful chemical manufactures.
Part of my job scope back then was also handling bioremediation works at oilfield and refineries.
 For this, we were required to formulate special chemical that can detoxify and in-capsulate heavy metals and hydrocarbons when necessary. Hence, we turned to our bio chemical manufactures alliances which helped us complete our biochemical ingredients.
For this, we were required to formulate special chemical that can detoxify and in-capsulate heavy metals and hydrocarbons when necessary. Hence, we turned to our bio chemical manufactures alliances which helped us complete our biochemical ingredients.It is very important to know where to outsource for the chemicals and biochemicals that you need for either research or real industry application. One of my favourite source to seek for such information is sourcepoint.com. It is a global business to business product and service sourcing directory based on the United Nations Standard Products and Services Codes which provides an accurate efficient classification of goods and services. From this site, I am able to directly contact manufactures and distributors of almost anything worldwide.
Currently, as a Ph.D in chemical engineering student studying reaction/catalysis engineering and using acid catalyst, I can efficiently search for the right catalyst from all over the globe. For example, under the “Chemicals including Bio chemicals and gas materials”, I can find “Acid Catalysts”. So, I just click at “Acid Catalysts” and I will get a row of results on companies offering acid catalysts.
I’m not sure about you and what products you are interested in. If you are in need of any chemicals, why not search it yourself there.
Labels: Chemical, Chemical Engineer, Chemical Engineering, Chemistry
posted by Kipas Repair JB @ 12:58 AM,
,
![]()
My wife just submitted her Ph.D thesis
Friday, April 30, 2010
After years of research, abundance of perseverance, unlimited patience, finally, my wife has successfully submit her Ph.D thesis yesterday. In case you wonder, she's studying the fundamental of Progressive Freeze Concentration. This area of study falls under Separation Engineering, Chemical Engineering.
The joy of completing and submitting her thesis doesn't mean that the battle is over. (it's actually 90% over). She has to defend her thesis in an oral presentation (viva) soon to take place. But at least, a huge chunk of the task is over. Supported with some publication in international and national journals, conferences, book chapters, I hope and most importantly believe she'll make it. On top of that, she has also managed to patent her research/design and that's a big thing. Not only that, the fact that foreign companies and institution searched and consulted her ecause of her research expertise make me believe that she'll get her doctorate soon. Let's all hope and pray that she'll make it smoothly without much pain.

As for me, it is now my second semester and I strongly believe doing Ph.D is like a war in a battle field or Star Wars!!! The most important element is to be able to plan the research activities wisely. Like a general in a battle, we need to plan the best strategy to fight and later terminate our enemies. We need to be able to utilize all resources available intelligently. We need to know when to attack and when to sit back and defend ourselves.
Well, I can't say much now about it as I'm still doing it. I may ask my wife to share some of her sweet and sour experiences. It may be helpful for those who are still working on his/her postgraduate studies and those who plan to do so.
What do you think of that?
Photo credited to http://www.copylobby.com/Binding/Thesis%20Binding.htm
Labels: Chemical, Chemical Engineer, Chemical Engineering, phd, Study
posted by Kipas Repair JB @ 2:16 PM,
,
![]()
Natural Memory Foam Versus Synthetic Memory Foam
Sunday, March 14, 2010
As a normal human being, we need to sleep few hours a day. That includes me and I never care where or on what I sleep on. The most important thing is I can sleep peacefully for few hours and revive my energy before facing a new fresh day for new life and work.
However, I learn something important two days ago which imperatively associates with our health and sleeping activity. I came across a new terminology which is called memory foam. I have not heard about it before, so as a chemical engineer, it really caught my interest to learn and research more about it. Do you know what memory foam is? Basically, memory foam is polyurethane with additional chemicals increasing its viscosity and density. It is also often referred to as visco-elastic polyurethane foam.
 I believe a lot of the mattresses that we use nowadays are made of memory foams. To be specific, they are made of synthetic memory foams. Do we care? Honestly I did not bother about it. Do we know the risk and danger of it? Since, I did not care, I was not aware of any treat it has. However, I was very surprised after learning the potential risk from synthetic memory foams or shall I call it mattress chemical?!
I believe a lot of the mattresses that we use nowadays are made of memory foams. To be specific, they are made of synthetic memory foams. Do we care? Honestly I did not bother about it. Do we know the risk and danger of it? Since, I did not care, I was not aware of any treat it has. However, I was very surprised after learning the potential risk from synthetic memory foams or shall I call it mattress chemical?!It is a fact that toxins can now be found in nearly all common household products; from carpets to microwaves, paints, couches, mattresses and baby cribs to children's clothing. Many of these toxins accumulate in the body and are never expelled. Unfortunately, normal synthetic memory foam, yes the one we use to sleep on, also contains some level of toxic and may contribute to the level of toxic in our body.
When you sleep, your body regenerates and recharges itself. At this time, prolonged exposure to poisonous volatile organic compounds and toxic chemicals when your body is in this vulnerable state should not be taken lightly. Now, you really need to pay special attention on this matter.
New Mattress / Bed Illness Report PageThough, this information is not widely exposed, it has appeared in a TV news. Check out the following video which shares how serious and how much of chemicals do we have in our blood.
Reports of health problems linked to chemicals in new mattresses.
Unlike mattresses of 40+ years ago, mattresses today are manufactured with increased amounts of petroleum based compounds in the form of foams, plastics, and a variety of volatile chemicals (including controversial flame retardants). Research and personal accounts suggest people can become ill by the repeated and continuous exposure to these low level chemicals during the sleep process. This is especially true for people who have lower levels of liver detoxification enzymes known as cytochrome P-450 (thereby allowing chemicals to build up in their blood to higher than normal levels). Since research now shows these chemicals are in fact evaporating from the materials used to make modern day beds and that some are carcinogenic and mutagenic compounds, the next important step is to publicize any personal accounts of illnesses people develop immediately following a new bed/mattress purchase. Health effects caused by petroleum chemical exposure can weaken or damage the immune and nervous system. Interestingly, autoimmune disorders have also been linked with exposure to petroleum-based chemicals and have been found to be the underlying etiology of many common health problems today (soft tissue damage, arthritis, etc).
Source: http://www.chem-tox.com/beds/reports/index.htm
So, how bad, how much chemicals and what chemicals are there inside synthetic memory foam? I can’t answer how much chemicals are there inside synthetic memory foams, but typical synthetic memory foam contains the following chemicals:
• 1,1,1, 2-Tetrachloroethane
• Acetone
• Dimethylformamide
• Methyl benzene (toluene)
• Methylene dianiline
• toluene–neoprene
• Vinilideine chloride
Those chemicals are readily exposed to us and we cannot afford them to penetrate our body in anyway. If synthetic memory foam is that bad, what should you do?
Simply switch to natural memory foam. Natural memory foam contains zero toxic chemicals. It only contains natural ingredients such as:
• Cone essence
• Emulsion of Hevea brasiliensis milk in water
• Fats
• Hevea brasiliensis milk
• Hydrolyzed corn
• water
To view complete list of chemicals in various type of synthetic memory foams and natural memory foams, you can refer to myessentia.com/research/glues-toxic-components.
This information, I hope has exposed the real thing and it is now totally up to you to decide on the best for you and your family. The best option for mattresses is those made from natural memory foam.
Labels: Chemical, Chemistry, Learning Curve, renewable, Research, Technology
posted by Kipas Repair JB @ 7:00 PM,
,
![]()
What's A Gas Chromatography?
Sunday, May 03, 2009
I believe some chemical engineers know about gas chromatography and some may heard but don't know about it. Some others may don't have any idea on what a GC is.
I have personally used a gas chromatography during my masters between 2000-2002. I used a Gas Chromatography Thermal Conductive Detector (GC-TCD). A GC-TCD is used to analyzed gaseous products. Since I'm going to pursue my pH.D this coming July, I need to refresh my memories and skills on how to use the GC and also the software. In my case, the software is Chemstation while the GC that we use in our lab is Hewlett Packard Agilent model 6890. What's a GC?
What's a GC?
Gas-liquid chromatography (GLC), or simply gas chromatography (GC), is a common type of chromatography used in organic chemistry for separating and analyzing compounds that can be vaporised without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture (the relative amounts of such components can also be determined). In some situations, GC may help in identifying a compound. In microscale chemistry, GC can be used to prepare pure compounds from a mixture (definition taken from en.wikipedia.org/wiki/Gas-liquid_chromatography).
It's not going to be easy to explain about GC in a short post like this. A GC short course will take 3 days at least and will cost a lot. Hence, I may introduce a little back ground on GC and later explain more in stages. For an introduction, I share this video which will provide some background idea on what GC is all about.
Note: This is an education video from the Royal Society of Chemistry on gas chromatography using a flame ionisation detector (FID) with a brief mention of gas chromatography mass spectrometry (GCMS). From the "Modern Instrumental Techniques for schools and colleges" DVD. For more information on the Chemistry for our Future programme please visit http://www.rsc.org/CFOF (C) Royal Society of Chemistry.
Check out my story on my GC adventure...
....................................................................................................................................................
Recommended: Free Technical / Engineering Magazine.
Join Chemical Engineer Rocks FACEBOOK Group...
Labels: Chemical, Chemical Engineer, Equipments, Learning Curve
posted by Kipas Repair JB @ 12:35 PM,
,
![]()
Cool Chemical Slideshow
I stumbled upon an interesting blog called AKA Bogor "Chemistry Corner" and found an interesting chemical slide show created by the owner. It's a good way to show and share chemical structures or any form of illustration to your audience in a website or presentation. Check it out:
To make your own slideshow, visit photobucket.com/slideshows
....................................................................................................................................................
Recommended: Free Technical / Engineering Magazine.
Join Chemical Engineer Rocks FACEBOOK Group...
Labels: Chemical, Learning Curve
posted by Kipas Repair JB @ 12:28 PM,
,
![]()
Periodic Table of Elements
Tuesday, February 24, 2009
I have heard about them (Periodic Table of Elements video) last end of last year. They have created a very interactive way of learning the periodic table - using youtube.com. They explained all the elements in the periodic table via video and update the elements video content from time to time. If you haven't heard about them or haven't watch sample of the periodic table elements, feel free to watch some of the selected elements below. For your information, I have watched mercury, hydrogen, helium, lithium and seaborgium (seaborgium, Sg is has the simplest video so far possibily because it is a very rare element).
So, what is Periodic table (of elements)? Well, the periodic table of the chemical elements is a tabular method of displaying the chemical elements. Although precursors to this table exist, its invention is generally credited to Russian chemist Dmitri Mendeleev in 1869. Mendeleev intended the table to illustrate recurring ("periodic") trends in the properties of the elements. The layout of the table has been refined and extended over time, as new elements have been discovered, and new theoretical models have been developed to explain chemical behavior. (Source: Wikipedia)
Personally, I have not yet seen all the elements but I'm going to watch all the periodic elements video soon. What about you?
Be the best chemical engineer you could be. Learn something about chemical engineering that's not inside your textbook. Subscribe to the content of this blog.
Labels: Chemical, Learning Curve, Video
posted by Kipas Repair JB @ 10:12 PM,
,
![]()
The Function of Magnesium in Our Life
Tuesday, January 13, 2009
The following article is written by Kevin McNabb. For more details about him, please check at the end of this article.
Magnesium, an alkaline earth metal, is the ninth most abundant element in the universe by mass. It constitutes about 2% of the Earth's crust by mass, and it is the third most abundant element dissolved in seawater.
Magnesium is the 11th most abundant element by mass in the human body; its ions are essential to all living cells. The free element (metal) is not found in nature. Once produced from magnesium salts, it is now mainly obtained by electrolysis of brine and is used as an alloying agent to make aluminium-magnesium alloys, sometimes called "magnalium" or "magnelium".
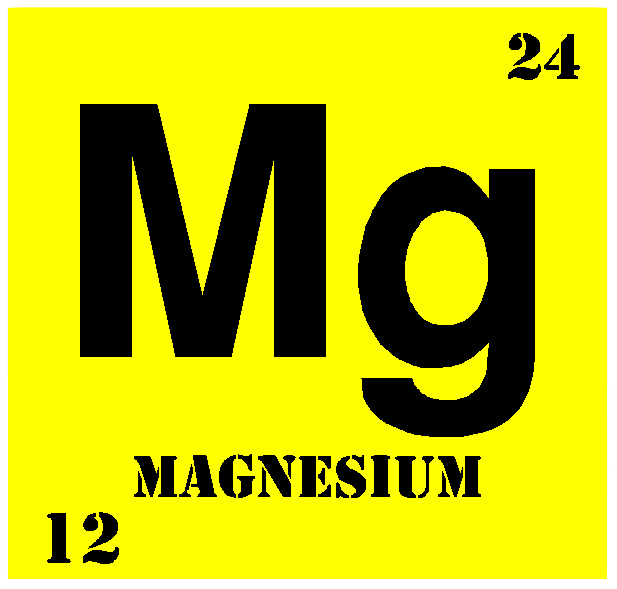 A balance of magnesium is vital to the well being of all organisms. Magnesium is a relatively abundant ion in the lithosphere and is highly bioavailable in the hydrosphere. This ready availability, in combination with a useful and very unusual chemistry, may have led to its usefulness in evolution as an ion for signalling, enzyme activation and catalysis. However, the unusual nature of ionic magnesium has also led to a major challenge in the use of the ion in biological systems. Biological membranes are impermeable to Mg2+ (and other ions) so transport proteins must facilitate the flow of Mg2+, both into and out of cells and intracellular compartments.
A balance of magnesium is vital to the well being of all organisms. Magnesium is a relatively abundant ion in the lithosphere and is highly bioavailable in the hydrosphere. This ready availability, in combination with a useful and very unusual chemistry, may have led to its usefulness in evolution as an ion for signalling, enzyme activation and catalysis. However, the unusual nature of ionic magnesium has also led to a major challenge in the use of the ion in biological systems. Biological membranes are impermeable to Mg2+ (and other ions) so transport proteins must facilitate the flow of Mg2+, both into and out of cells and intracellular compartments.
Key Functions of Magnesium
- Formation of bone - About two thirds of magnesium resides in bone. Researchers have found that this bone magnesium plays two very distinct roles in the support of health. Some of this magnesium contributes to the physical structure of bone, being part of the bones crystal lattice, its "scaffolding", along with calcium and phosphorous. The other portion of magnesium is found on the bone surface and acts as a storage site for magnesium that the body can draw upon during times of inadequate magnesium intake.
- Relaxation of nerves and muscles - Magnesium and calcium act in conjunction to help to regulate nerve and muscle tone. In many nerves, magnesium serves the function of being a chemical gatekeeper; when there is enough magnesium around, calcium is blocked from rushing into the nerve cell and activating the nerve and the nerve is kept in a state of relaxation. If dietary magnesium is inadequate, the gate blocking can fail and the nerve may become overactivated. When certain nerve cells are overstimulated, they send too many messages to the muscles causing them to overcontract. This series of events helps to explain why magnesium deficiency can cause muscle soreness, tension, spasms, cramps and fatigue.
- Other functions of magnesium - Many chemical reactions in the body involve the presence of enzymes, proteins that help to catalyze chemical reactions. Since magnesium plays a role in over 300 different enzymes, its physiological functions are very extensive and include (but are certainly not limited to) being involved in protein, carbohydrate and fat metabolism, storage of energy in muscle cells and the proper functioning of genes. Since the metabolic role of magnesium is so ubiquitous, it is difficult to identify a body system that would not be affected by a magnesium deficiency. The digestive system, endocrine system, cardiovascular system, nervous system, muscles, kidney, liver and brain all rely upon magnesium to carry out their metabolic functions.

Food Sources of Magnesium
- Pumpkin and squash seed kernels, Brazil nuts, Bran ready-to-eat cereal (100%), Halibut, Quinoa, Spinach, Almonds, Spinach, Buckwheat flour, Cashews, Soybeans, Pine nuts, Mixed nuts, White beans, Pollock, walleye, Black beans, Bulgur, Oat bran, Soybeans, Tuna, Artichokes, Peanuts, Lima beans, Beet greens, Navy beans, Tofu, Okra, Soy beverage, Cowpeas, Hazelnuts, Oat bran muffin, Great northern beans, Oat bran, Buckwheat groats, Brown rice, Haddock
The magnesium content of plants varies considerably with how much magnesium is in the soil where the plants are grown.
Much of the magnesium content in food is lost during processing - milling removes approximately 59% of the magnesium from whole wheat.
Cooking foods in water also causes magnesium to leach out during the cooking process.
Recommended Daily Usage
- 0-6 months - 50mg
- 6-12 months - 70mg
- 1-10 years - 150-250mg
- 11-18 years - 300-400mg
- 18 years + - 300-400mg
- pregnant / lactating - + 150mg
- Therapeutic Range: 50mg - 2500mg+
Nutritional Safety
- Deficiency (Not enough Magnesium): Poor dietary intake of magnesium is a common cause of deficiency as are gastrointestinal tract problems such as malabsorption, diarrhea and ulcerative colitis. Physical stresses such as trauma, cold stress and surgery can also contribute to a magnesium deficiency as can kidney disease and alcoholism. The symptoms of magnesium deficiency can impact many physiological processes since this mineral plays such a wide variety of roles in the body. Common symptoms involve changes in muscle and nerve function such as muscle weakness, spasm and tremor. Since the heart is a muscle it can also experience compromised functioning concomitant with magnesium deficiency which can result in arrhythmia, irregular contractions and increased heart rate. Softening and weakening of bone can be the result of a deficiency since magnesium plays an important role in the maintenance of bone structure. Included among other symptoms of magnesium deficiency are imbalanced blood sugar, elevated fats in the bloodstream, elevated blood pressure, headaches, seizures, depression, nausea, vomiting and lack appetite.
- Toxicity (Too much Magnesium): Diarrhea is the most commonly experienced toxicity symptom associated with high intake of magnesium. It is most frequently seen when magnesium is taken as a dietary supplement rather than from food sources. While diarrhea can occur at lower supplemental doses, in research studies the doses of magnesium associated with diarrhea range from 1,000-5,000 miligrams. In addition, generalized symptoms such as increased drowsiness or a sense of weakness may be attributable to magnesium toxicity.
My next article entitled "The Function of Selenium in our Life" will examine the role of Selenium in good nutrition.
See you on the Beaches of the World,
Kevin McNabb
Kevin McNabb is the Chief Executive Officer of Toronto-based http://www.GlobalHealthMgmt.com a health, wellness, beauty, and personal care e-commerce site. He is a network marketer, author, freelance writer, and offers seminars and Internet training programs on personal development for the network marketing industry. For more information on the topic of this article, please see http://www.globalhealthmgmt.com/Vitamins_Supplements.html
_________________________________________
Get FREE Technical Magazines - Chem-Eng.Tradepub.com.
> Do you understand about ORGANIC FOOD... Check out about it...
>> Join Chemical Engineer Rocks FACEBOOK Group...
Be the best chemical engineer you could be. Learn something about chemical engineering that's not inside your textbook. Subscribe to the content of this blog.
Labels: Chemical, Learning Curve
posted by Kipas Repair JB @ 9:01 PM,
,
![]()
The Author

I’m Zaki. I used to be a project, process and chemical engineer. Few years ago I successfully became a Chartered Engineer (IChemE) and Professional Engineer (BEM). I'm now employed as a chemical engineering educator/researcher/consultant. Hope you like reading my blog. I welcome any feedback from you. My email: zaki.yz[alias]gmail.com. TQ!



